Navigation
Explanation
1. What is drug-drug interaction?
Drug-drug interaction (DDI) is broadly described as a change in the effect of one drug due to the presence of another drug. Most interactions can seriously affect efficacy and safety drug profiles and have been a significant cause of adverse drug interactions (ADRs). DDIs can be divided into pharmaceutical, pharmacokinetic (PK) interactions, and pharmacodynamic (PD) interactions. Pharmaceutical interactions occur due to physical or chemical incompatibilities, which are often out of the conventional scope of DDIs. PK interactions could influence the drug disposition process to change the free-drug concentration at the target site, including absorption, distribution, metabolism, and excretion (ADME). While PD interactions usually are synergistic or antagonistic, which can occur by interacting with the same therapeutic targets, involving in different signaling pathways, or causing different pharmacological responses. We mainly focus on the latter two kinds of DDIs in this work and the details of these subclasses are elaborated.
2. What can DDInter do?
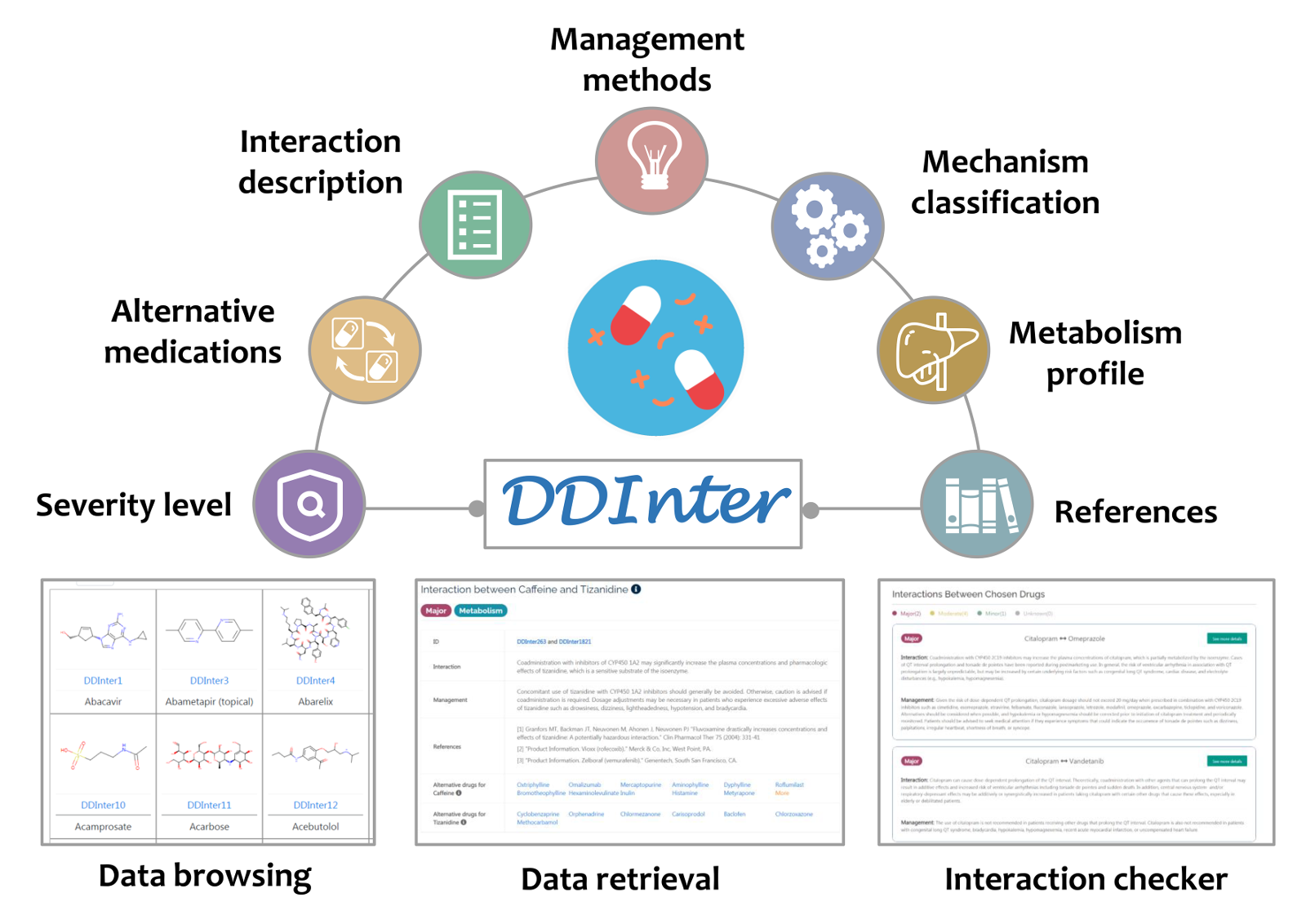
For physicians and pharmacists:
- Carry out dose adjustment according to the interaction descriptions
- Judge if the advantages overweight disadvantages based on the risk level
- Gain drug replacement guidance from the alternative medications
- Identify dangerous combinations to prevent potential ADRs timely
For data scientists:
- Obtain valuable interaction data as the resources for the detection of potential DDIs
- Employ DDInter to validate the performance of DDI prediction tools
3. Criteria for mechanism annotation
3.1 Absorption
Absorption-related drug interactions are commonly associated with various mechanisms, with most cases hindering and very few promoting. First, decreased absorption may be secondary to chelation with a cation such as calcium or iron. Second, absorption may be decreased when the dissolution of the medication is highly dependent on gastric pH. Third, intestinal absorption may be influenced by inhibition or induction of the p-glycoprotein efflux transporter in the intestinal epithelium.
- Absorption-related drug interactions are commonly associated with various mechanisms, with most cases hindering and very few promoting. First, decreased absorption may be secondary to chelation with a cation such as calcium or iron. Second, absorption may be decreased when the dissolution of the medication is highly dependent on gastric pH. Third, intestinal absorption may be influenced by inhibition or induction of the p-glycoprotein efflux transporter in the intestinal epithelium
- The absorption of itraconazole is pH-dependent and may be impaired when gastric pH is increased after antacid administration such as proton pump inhibitor omeprazole.
- The macrolides may potentiate digoxin toxicity. The inhibition of the P-gp transporter in the intestine is the mechanism of this interaction, as digoxin is a p-glycoprotein substrate and the macrolides may inhibit this transporter.
3.2 Distribution
The distribution of medications into tissues is mediated by drug influx and efflux transporters and influenced by protein binding as only the free fraction will be able to penetrate across tissue membranes. Distribution-related drug interactions include competing for the plasma protein binding sites, changing the free-drug fraction, or influencing the distribution in some tissues and organs, etc.
- Methotrexate leads to hematological toxicity when coadministered with acetylsalicylic acid. This interaction occurs due to decreased clearance of methotrexate through competition with acetylsalicylic acid, resulting in methotrexate being displaced from its plasma protein binding site.
- Coadministration with drugs that reduce catecholamine uptake or deplete catecholamine stores may interfere with iobenguane I-131 uptake into neuroendocrine tumors such as pheochromocytoma and paraganglioma that express high levels of norepinephrine transporter on their cell surfaces.
3.3 Metabolism
Metabolic interactions are mostly due to CYP450 isoenzymes and are responsible for up to 40% of DDIs found in patients. Medications interacting with the CYP450 system can be classified as substrates, inhibitors, or inducers.
- The co-administration of zidovudine with valproic acid causes increased oral bioavailability of zidovudine, due to reduced metabolism of zidovudine by glucuronidation. This interaction may increase the risk of adverse effects, including severe anemia and hematologic toxicity.
- Rifampin, due to its enzyme-inducing activities, may increase the metabolism of lamotrigine, reducing the AUC by up to 40%. This can result in decreased efficacy in preventing and controlling seizures.
3.4 Excretion
As the main excretion organ, most excretion-related interactions occur in the kidney, including affecting the PH of renal tubule fluid, inhibiting the tubular secretion, changing the kidney blood flow, etc.
- Probenecid is a uricosuric and renal tubular blocking agent that inhibits the tubular secretion of penicillin and usually increases penicillin plasma levels by any route the antibiotic is given. Probenecid is indicated to be given as an adjuvant to therapy to penicillin to enhance their plasma levels, and concomitant use has been associated with increased penicillin-related adverse reactions.
- Diuretics may increase serum lithium concentration. Diuretics affect filtration rates and affect electrolyte exchange in the nephron. By reducing sodium reabsorption thiazide diuretics can increase the reabsorption of lithium and result in increased serum lithium concentrations.
3.5 Synergy
The synergistic effect occurs when the overall effect of the drug combination is greater than additive. The synergistic effect can be beneficial or adverse, which is decided by the pharmacological characteristics of drug combinations.
- The additive effects between benzodiazepines and opiates can lead to respiratory depression and possibly cause coma and death. The risk of respiratory depression, coma and death is important to note. This interaction occurs due to the co-location of GABA and opiate receptors in the central nervous system, and cross-reactivity and common pathways of intracellular transduction for these agents.
- Following furosemide injection, there was a rapid and reversible decrease in the endocochlear potential and eighth nerve action potential with a more gradual decrease of the endolymph potassium concentration, leading to transient or permanent ototoxicity. As aminoglycoside antibiotics have the potential to cause ototoxicity, furosemide may increase the ototoxic potential of aminoglycoside antibiotics, especially in the presence of impaired renal function.
3.6 Antagonism
The antagonistic effect occurs when the overall effect of the drug combination is less than additive. Sometimes antagonistic interactions are used to alleviate the harmful effects of certain medications clinically, while they may also offset the pharmacological effects of the drugs taken together.
- The concomitant administration of an opioid antagonist with an opioid can result in the mitigation of both classes of medications' therapeutic effects for the patient. While the pharmacological effect of the opioid antagonist to treat or manage opiate abuse or overdose can be inhibited, at the same time the legitimate pharmacological effect of any opioids administered for the genuine management of pain can also be decreased.
- Some beta-blockers, such as propranolol, may antagonize the bronchodilatory, hypotensive, and tachycardic effects of isoproterenol. The mechanism is blockade of beta-adrenergic receptors, which leads to bronchoconstriction, vasodilation, and increased heart rate. Beta-blockers have been used successfully to treat catecholamine or isoproterenol-induced tachyarrhythmias.
3.7 Others and Unknown
The expanded descriptions of some interactions were ambiguous, which made it difficult work to identify the concrete categories. Therefore, these interactions were annotated with ‘Others’. In addition, the DDIs collected from the article published in Sci Transl Med were lack of mechanism descriptions, and thus these DDIs were annotated with 'Unknown'.
4. Criteria for severity annotation
Categories of severity were accepted as suggested by DRUGDEX and other similar resources:
- Major: The interactions are life-threatening and/or require medical treatment or intervention to minimize or prevent severe adverse effects.
- Moderate: The interactions may result in exacerbation of the disease of the patient and/or change in therapy.
- Minor: The interactions would limit the clinical effects. The manifestations may include an increase in frequency or severity of adverse effects, but usually they do not require changes in therapy.
- Unknown: The DDIs collected from the article published in Sci Transl Med were lack of mechanism descriptions, and thus the severity classifications of these DDIs were annotated with 'Unknown'.